 |
 |
- Search
| Neurointervention > Volume 19(1); 2024 > Article |
|
Abstract
Vertebral artery stump syndrome (VASS) is a rare condition that can cause posterior circulation ischemic stroke due to occlusion of the ipsilateral vertebral artery (VA) orifice, resulting in blood flow stagnation and embolus formation. Although there is no established treatment for this condition, we observed 3 cases of VASS out of 326 acute ischemic stroke cases at a single institution from April 2021 to October 2022. Despite the best possible antithrombotic treatment, all 3 patients had recurrent ischemic strokes. One patient underwent drug-eluting stenting of the VA orifice to relieve occlusive flow. The other 2 patients received coil embolization, which resulted in the disappearance of their culprit collateral flow. None of the patients had recurrent ischemic strokes after endovascular intervention. Based on our observations, stenting and coil embolization are effective methods for preventing future recurrences of VASS.
Vertebral artery stump syndrome (VASS) is a rare disease resulting in posterior circulation ischemic stroke caused by an embolus derived from stagnating blood flow after vertebral artery (VA) orifice occlusion [1]. Recurrence can be fatal, sometimes causing basilar artery occlusion [2]; however, no optimal treatment has been established.
VASS was first described by Nguyen et al. [3] in 2008 and defined by Kawano et al. [1] in 2013. Since then, there have been a few reports on medication and endovascular treatment (EVT) [1-11], such as percutaneous transluminal angioplasty with/without stenting (PTA/S) and coil embolization for VASS. However, no studies have addressed in what cases PTA/S and coil embolization are effective. This case series will focus on 3 patients who underwent EVT to prevent further ischemic stroke caused by VASS.
Between April 2021 and October 2022, we retrospectively reviewed patients diagnosed with VASS at our institution. The criteria for diagnosis of VASS were as follows: (1) the occurrence of acute ischemic stroke in the posterior circulation; (2) acute occlusion of the VA orifice; (3) the existence of distal antegrade blood flow in the ipsilateral VA despite its orifice occlusion; and (4) the absence of any other cause of ischemic stroke [1]. Of 326 consecutive acute ischemic stroke cases, 3 (0.9%) were diagnosed with VASS during the study period. All patients diagnosed with VASS had experienced repeated ischemic strokes and received EVT. The following section provides details on the indication and selection of EVT in these 3 specific cases that focus on technical aspects. For the article’s publication, we obtained approval from a relevant institutional review board and informed consent from patients and their families.
An 80-year-old male had dizziness and dysarthria. On admission, diffusion-weighted imaging (DWI) revealed an acute ischemic stroke in the left cerebellar hemisphere. Digital subtraction angiography (DSA) showed left VA orifice occlusion. Collateral flow via a deep cervical artery reconstructed the left VA at the C3 and C4 levels. Antegrade and stagnant blood flow existed in the left VA, leading to the diagnosis of VASS.
The patient had received dual antiplatelet therapy for peripheral artery disease before admission. Due to extensive infarction, no antithrombotic therapy was administered after admission because of concerns about hemorrhagic infarction. On day 3, he had hemorrhagic infarction at the left posterior inferior cerebellar artery area and right superior cerebellar artery area. On day 5, he was treated with edoxaban 60 mg after we confirmed that there was no exacerbation of hemorrhagic infarction; however, on day 8, the patient showed exacerbation of dysarthria and a recurrence of infarction in the right cerebellar hemisphere of the superior cerebellar artery area. In this case, anticoagulation therapy could not prevent recurrent ischemic stroke and the patient received PTA/S. PTA/S was chosen over coil embolization for 2 reasons: First, there was poor collateral circulation in the basilar artery, with a hypoplastic contralateral VA ending in the posterior inferior cerebellar artery. Second, despite being occluded, the left VA orifice was still visible.
Before PTA/S, the patient was administered a loading dose of 200 mg aspirin and 20 mg prasugrel. First, proximal flow control was performed in the left subclavian artery using 9-Fr Optimo 90 cm (Tokai Medical Products, Inc.), causing the flow of the left VA to reverse. Second, the occlusive lesion was carefully pierced using a CHIKAI14 (Asahi Intecc Co., Ltd.) and SL-10 (Stryker). Spider FX 4 mm (Medtronic) was guided to the left VA at the C2 level using an over-the-wire technique. Third, balloon angioplasty with Gateway 2.0×12 mm (Stryker) at 6 atm 90 seconds, Coyote ES 2.5×30 mm (Boston Scientific) at 6 atm 90 seconds, and 9 atm 90 seconds was performed; however, the stenotic lesion recoiled. Finally, a coronary stent, Synergy XD 3.0×24 mm (Boston Scientific) was placed in the left VA orifice at 8 atm 100 seconds. Reperfusion and disappearance of stagnancy in blood flow in the left VA were confirmed (Fig. 1).
After EVT, he was treated with argatroban for 2 days and dual antiplatelet therapy with aspirin 100 mg and prasugrel 3.75 mg for 3 months; after that, prasugrel monotherapy was continued. The postoperative course went well. He had no recurrence and was transferred to a rehabilitation hospital on day 33. He experienced no further events during the 6-month follow-up period.
A 62-year-old male had dizziness and disturbance. DWI revealed a high-intensity signal in the bilateral cerebellar hemisphere on admission. DSA showed right VA orifice occlusion. On day 1, he required suboccipital decompressive craniectomy because of acute hydrocephalus. On day 7, he had new dysphagia and ischemic stroke at the right medulla. On day 9, he had recurrent infarction at the left superior cerebellar artery area. Repeated DSA showed right VA orifice occlusion and collateral flow via the ascending cervical artery at the C3/C4 levels and the occipital artery at the C1 level. He was diagnosed with VASS at this time.
In this case, we hesitated at first to provide antithrombotic therapy due to concerns about hemorrhagic infarction. Moreover, as antithrombotic therapy alone was insufficient, we administered EVT without medical treatment. Following recurrence, the patient underwent coil embolization of antegrade VA flow due to collateral arteries. We chose to perform coil embolization over PTA/S for 2 main reasons: First, there was a well-developed contralateral VA. Second, the VA orifice was not visible.
First, a 6-Fr FUBUKI 90 cm (Asahi Intecc Co., Ltd.) was placed in the left VA. Second, an SL-10 (Stryker) and Traxcess (Terumo) were placed at the right VA via the union. Finally, 10 coils were placed in the right VA of the C1 and C2 levels. The collateral arteries disappeared, and the stagnancy in blood flow in the proximal right VA was eliminated (Fig. 2).
After EVT, he was treated with edoxaban 60 mg. He was transferred to a rehabilitation hospital on day 35. He had no recurrence during the 4-month follow-up period.
A 66-year-old male presented with bilateral hemianopsia. DWI showed high-intensity in the bilateral occipital lobe. Computed tomography angiography revealed left VA orifice occlusion and collateral flow via the well-developed deep cervical artery to C1/C2 levels and ascending cervical artery to C2/C3 levels. He was treated with 100 mg aspirin. However, on day 4, he had a recurrence in the left cerebellar hemisphere. The medication was changed from aspirin to 300 mg dabigatran, but on day 6, a new infarction was observed in the right occipital lobe. DSA revealed left VA orifice occlusion and a thrombus near the inflow section of the ascending cervical artery.
In this case, antiplatelet and anticoagulation therapy failed to prevent recurrent ischemic stroke and the patient underwent EVT. Coil embolization was chosen as the preferred treatment alternative for this patient due to 2 factors. First, DSA indicated suspected thrombus formation, which could have led to distal embolization during the procedure. Second, the VA orifice was not visible. As the contralateral VA was not well-developed, we tried to place the coil at the proximal VA and preserve the well-developed deep cervical artery that supplied blood to the basilar artery during the procedure. Consequently, an 8-Fr FUBUKI 80 cm (Asahi Intecc Co., Ltd.) was placed at the left subclavian artery. A RADIFOCUS guidewire 035 (Terumo) was guided to the brachial artery as a buddy wire because the guiding catheter slipped easily into the aorta because of the low bifurcation of the VA. A Guidepost 3.2/3.4 130 cm (Tokai Medical Products, Inc.) was placed in the distal part of the deep cervical artery, and a GREACH 1.7/2.4 157 cm (Tokai Medical Products, Inc.) was placed in the VA. Coil embolization was performed to preserve the deep cervical artery, and the stagnancy in blood flow in the proximal VA disappeared (Fig. 3).
After EVT, he was treated with 300 mg dabigatran. On day 41, he was transferred to a rehabilitation hospital. He had no recurrence during the 1-year follow-up period.
We treated 3 patients with VASS through EVT, which was effective in preventing recurrent strokes. Although some reports have shown that dual antiplatelet and anticoagulant therapies are effective for VASS [1,9,10], others have reported cases that were refractory to these therapies [1,3]. Therefore, an appropriate medical therapy for VASS has not been established. In the presented cases 1 and 3, the administration of medical treatment with direct oral anticoagulants was ineffective because the patients subsequently had a recurrent stroke.
We identified 11 articles with 28 cases about “vertebral artery stump syndrome” published from January 2008 to September 2023 on PubMed [1-11]. Recurrence was observed in 7 out of 19 cases (37%) with only medical treatment [1-3,9-11] and in only 2 out of 11 cases (18%) with EVT [3-8]. Both groups included 2 patients who underwent medical treatment initially, but later required EVT due to recurrence [3]. Additionally, no acute recurrence was observed in the EVT group (Supplementary Table 1).
It needs to be noted that there may have been a selection bias against medical treatment because (1) VASS may be diagnosed only in cases of repeated recurrence and (2) it might be reported in cases where EVT was successful. However, our experience suggests that EVT may be effective in stopping recurrent strokes in cases where VASS is refractory to medical treatment, and further case series and prospective studies are warranted.
EVT, such as PTA/S and coil embolization, aims to eliminate the embolic source called the stump. PTA/S revascularizes the antegrade flow in the VA and has been selected recently (Supplementary Table 1). The advantages of PTA/S are its ability to maintain antegrade blood flow to the basilar artery and prevent the development of new collateral pathways to the ipsilateral VA. The disadvantages include the possibility of distal embolization, the difficulty of lesion cross in cases where the VA orifice is not visible, and the need for postoperative antiplatelet therapy. According to Zhang et al. [6], 2 patients who underwent PTA had recurrent cerebral infarction or transient ischemic attack. In a case where only PTA was performed in the initial EVT, close follow-up is required, and stenting may be considered on an elective basis.
In the Vertebral Artery Ischemia Stenting Trial, 48 patients with extracranial VA stenosis were treated with stenting, and no significant complications were observed [12], which also supports the safety of PTA/S in VASS.
In contrast, coil embolization occludes the stump supplied via collateral arteries. We considered that narrow collateral arteries can cause turbulence and thrombus formation; thus, coil embolization targets the inflow zone of these arteries. By eliminating the collateral arterial supply that causes embolus formation, it may be possible to prevent new embolization. The advantages of coil embolization are possible in cases where the VA orifice is not visible and does not cause distal embolization. The disadvantage of coil embolization is reduced antegrade blood flow to the basilar artery and the potential risk of new stumps in the distal part of the embolized VA. Although only 2 cases of coil embolization have been reported, no complications were observed. Similarly, there were no complications in cases 2 and 3. Coil embolization may be effective in cases where a large thrombus exists, such as the subacute phase.
In PTA/S, the protection method, choice of stent or balloon, and appropriate antiplatelet therapy are essential. The VA is narrower than the internal carotid artery, with much individual variation. Therefore, the devices should be considered in each case. Distal embolic protection devices, such as Spider FX and Filerwire EZ (Boston Scientific), can be an option. In case 1, a balloon-guiding catheter was inflated in the subclavian artery, and the flow of the VA was reversed. Thus, the combination of proximal protection is a good option for preventing distal embolization.
Drug-eluting stents are more helpful in preventing restenosis than bare metal stents [13]. In addition, the balloon expandable stent is helpful for accurate positioning in the VA orifice. When implanting the stent, the stent position should be finely controlled by expanding it like a dog bone. In case 1, a third-generation drug-eluting stent, Synergy XD, was used, and the stent was accurately placed. The appropriate duration of antithrombotic after VA stenting has not been established yet. Considering the data on coronary stenting, the duration of dual antiplatelet therapy after drug-eluting stent placement may be sufficient for 1–3 months [14].
The contralateral blood flow of the VA, access route, and extent of embolization are essential in the coil embolization of the stump. When eliminating antegrade blood flow in the culprit VA, we should avoid posterior circulation ischemic infarction due to hypoperfusion. There have been a few cases of balloon test occlusion in the VA, and the efficacy is not clear [15]. According to Zoarski and Seth [16], when retrograde blood flow via the contralateral VA fills the ipsilateral V4 segment of VA, the VA can be safely occluded without a balloon test occlusion. However, it is desirable to preserve collateral vessels that are not involved in VASS pathophysiology.
The target lesion of embolization should be considered in each case. In VASS, the collision of blood flow from collateral arteries may cause blood stasis and thrombus formation. In case 2, all ipsilateral collateral arteries were narrow; therefore, stump occlusion was performed for all ipsilateral collateral arteries. In case 3, the ipsilateral deep cervical artery was well-developed, and the thrombus formed in a more proximal VA around the inflow of narrow ascending cervical arteries. We considered that abundant blood flow from the deep cervical artery would not cause VASS pathophysiology; therefore, we preserved the flow from the deep cervical artery and embolized the proximal VA. To summarize the concept of our coil embolization, we preserve large blood vessels that may affect intracranial blood flow, while eliminating small collateral vessels that may lead to blood stasis.
Nguyen et al. [3] reported that after coil embolization, 1 patient received dual antiplatelet therapy for a year and switched to single antiplatelet therapy. The other received warfarin for a month and switched to single antiplatelet therapy. Both patients had no recurrence for over a year [2]. In cases 2 and 3, direct oral anticoagulant therapy (dabigatran and edoxaban) was continued, and no recurrence was noted. Single antithrombotic therapy may be sufficient to prevent recurrent strokes.
In conclusion, VASS is a rare condition wherein recurrent cerebral infarction occurs in the posterior circulation because of stagnancy in blood flow associated with the VA orifice occlusion [1]. EVT, such as PTA/S and coil embolization may prevent recurrent cerebral infarction by eliminating the stump. Methods of EVT should consider the time since onset, the degree of collateral arteries, contralateral VA development, and the availability of antithrombotic therapy.
SUPPLEMENTARY MATERIALS
Supplementary materials related to this article can be found online at https://doi.org/10.5469/neuroint.2023.00416.
Supplementary Table 1.
Summary of VASS cases that mentioned to recurrence after EVT in previous studies
Acknowledgments
The authors acknowledge Dr. Justin Turpin for obtaining the images and video for case 1.
Notes
Ethics Statement
For the publication of this article, we obtained approval from a relevant institutional review board. And we obtained informed consent of this publishment from the patients and their families.
Author Contributions
Concept and design: RS and TM. Analysis and interpretation: RS and TM. Data collection: RS and TM. Writing the article: RS. Critical revision of the article: RS and TM. Final approval of the article: RS, TM, YT, SH, DS, NH, TK, and YU. Statistical analysis: RS. Overall responsibility: RS.
Fig. 1.
Various findings of case 1 (an 81-year-old male). On days 1 (A), 5 (B), and 8 (C), diffusion-weighted imaging revealed the presence of recurring ischemic stroke of the superior cerebellar artery area. Based on the magnetic resonance angiography findings (D), the right vertebral artery (VA) appears to be the posterior inferior cerebellar artery end. Therefore, it was not reasonable to sacrifice the left VA. The left VA ostium was occluded, and antegrade blood flow was detected through the deep cervical artery at the C3/4 level (arrow) based on frontal (E) and lateral (F) views. VA occlusion was recanalized with angioplasty and stenting, as confirmed by frontal (G) and lateral (H) views. No recurring ischemic events were reported following the endovascular treatment procedure.
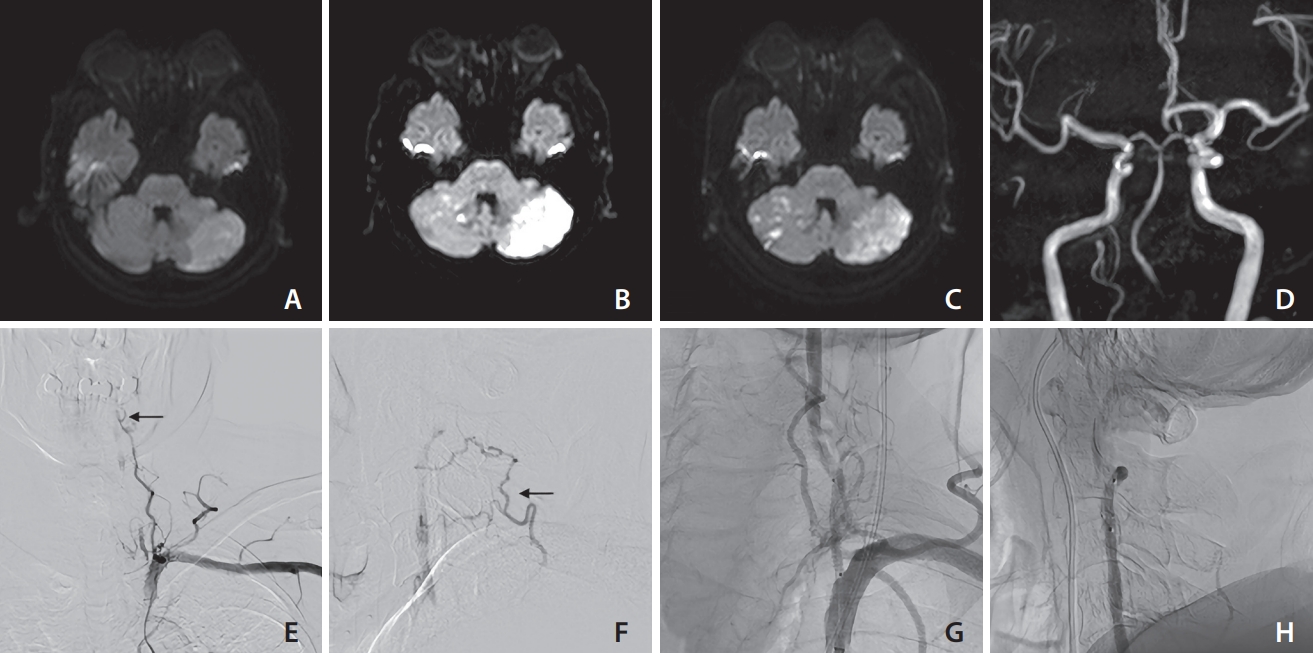
Fig. 2.
Various findings of case 2 (a 62-year-old male). On days 1 (A), 7 (B, C), and 9 (D), diffusion-weighted imaging revealed the presence of recurring ischemic strokes. Based on magnetic resonance angiography findings (E), the left vertebral artery (VA) appeared well-developed. The right VA ostium was occluded, and antegrade blood flow was detected through the ascending cervical artery at the C3/4 level (arrow) and occipital artery at the C1 level (arrow head), based on frontal (F) and lateral (G, H) views. Coil embolization in the inflow zone of the collateral arteries at the C1/2 level was performed via contralateral VA (I). The stagnancy in blood flow disappeared, as confirmed by frontal (J) and lateral (K, L) views. No recurring ischemic events were observed following the endovascular treatment procedure.
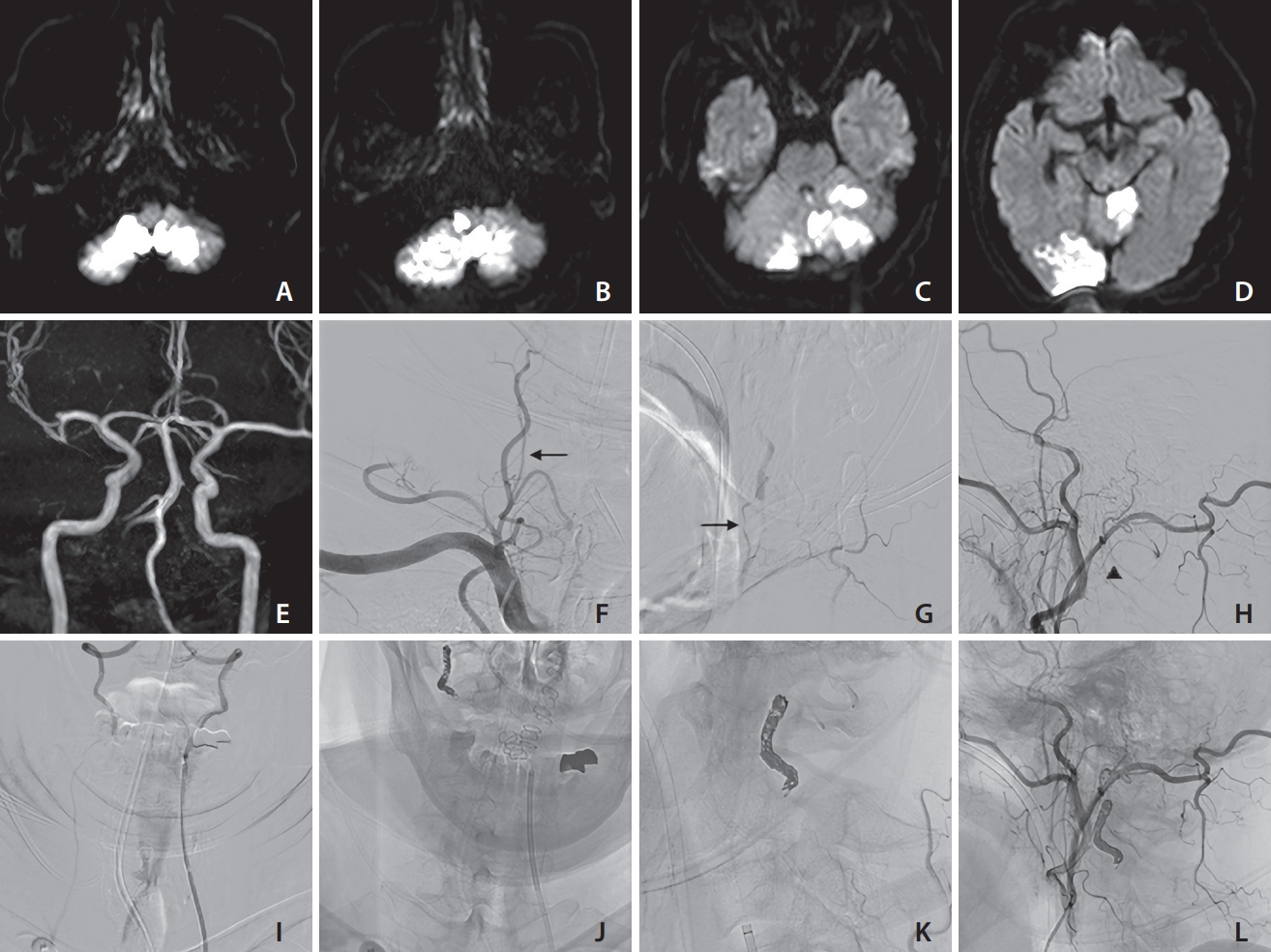
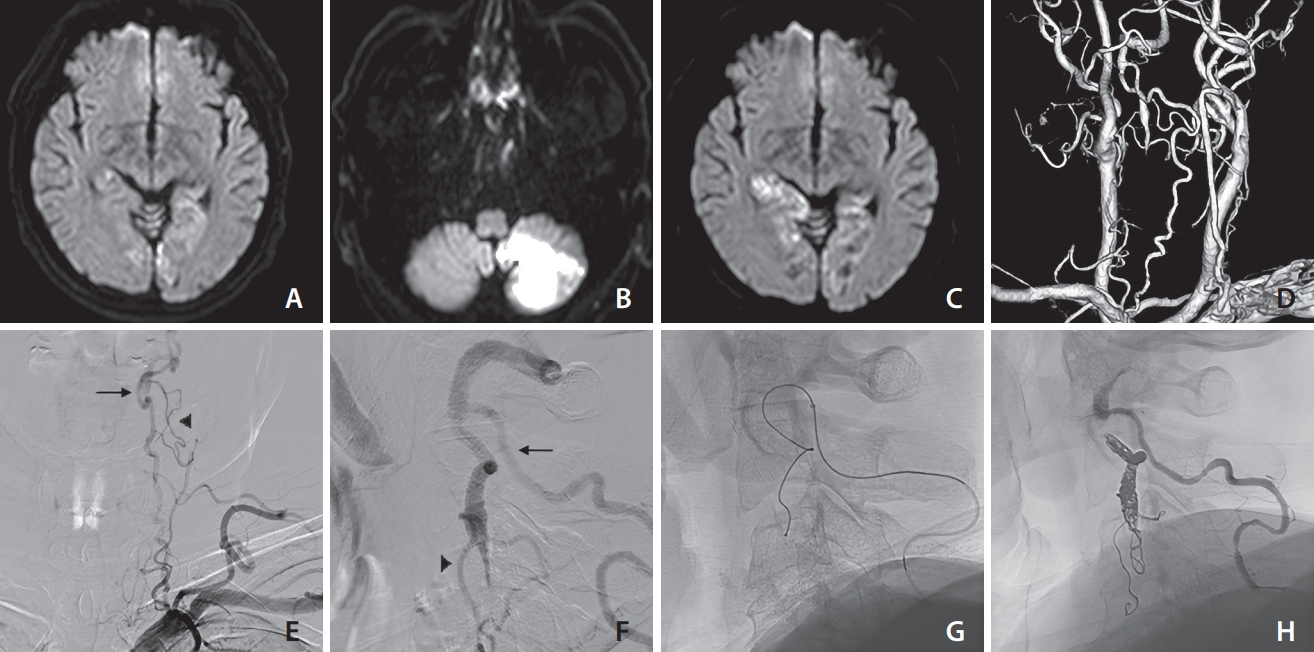
Fig. 3.
Various findings of case 3 (a 66-year-old male). On days 1 (A), 4 (B), and 6 (C), diffusion-weighted imaging revealed the presence of recurring ischemic strokes. Based on the posterior view (D) of computed tomography angiography, the left vertebral artery appeared occluded from the orifice and the deep cervical artery was well-developed. Antegrade blood flow was detected through the deep cervical artery at the C1/2 level (arrow) and ascending cervical artery at the C2/3 level (arrow head), based on frontal (E) and lateral (F) views. Coil embolization was performed at the distal end of the thrombus via the deep cervical artery (G). The stagnancy in blood flow disappeared, as confirmed by the lateral (H) view. No recurring ischemic events were observed following coil embolization.
REFERENCES
1. Kawano H, Inatomi Y, Hirano T, Yonehara T. Vertebral artery stump syndrome in acute ischemic stroke. J Neurol Sci 2013;324:74-79.


2. Yamano A, Nakai Y, Akutagawa K, Igarashi H, Tsukada K, Terakado T, et al. Fatal recurrent ischemic stroke caused by vertebral artery stump syndrome. Surg Neurol Int 2021;12:445



3. Nguyen TN, Raymond J, Mahmoud M, Weill A, Roy D, Guilbert F. Vertebral artery stump syndrome. J Neurol Neurosurg Psychiatry 2008;79:91-92.


4. Ji R, Li B, Xu Z. Retrograde recanalisation for vertebral artery stump syndrome: a case report. Stroke Vasc Neurol 2022;7:462-464.



5. Okamoto A, Nakagawa I, Kotsugi M, Yokoyama S, Yamada S, Park YS, et al. Endovascular vertebral artery orifice angioplasty for the prevention of acute ischemic stroke following vertebral artery stump syndrome. Surg Neurol Int 2022;13:382



6. Zhang W, Wang S, Li C, Wang Z, Yue F, Zhou J, et al. A case series and literature review of vertebral artery stump syndrome. Front Neurol 2022;12:770845



7. Oda K, Noda M, Ishibashi T, Kogiku M, Abe K, Kishi H, et al. Percutaneous transluminal angioplasty for suspected vertebral artery stump syndrome. Neuroradiol J 2020;33:520-524.



8. Maeoka R, Nakagawa I, Ohnishi H, Kuga Y, Nakase H, Ohnishi H. A thread of hope for successful revascularization for acute embolic basilar artery occlusion due to miserable vertebral artery stump syndrome. A technical report. J Clin Neurosci 2020;73:299-303.


9. Katayama M, Endo H, Matsuda M, Kamiyama K, Osato T, Nakamura H. Vertebral artery stump syndrome: a 7-year follow-up case report. Radiol Case Rep 2022;17:2923-2926.



10. Tempaku A. Cerebral angiography directly visualizes to-and-fro stream of vertebral artery stump syndrome. J Gen Fam Med 2017;18:462-463.



11. Suzuki M, Dembo T, Hara W, Tajima T, Yamashita M, Oji S, et al. Vertebral artery stump syndrome. Intern Med 2018;57:733-736.



12. Markus HS, Larsson SC, Kuker W, Schulz UG, Ford I, Rothwell PM, et al, VIST Investigators. Stenting for symptomatic vertebral artery stenosis: the vertebral artery ischaemia stenting trial. Neurology 2017;89:1229-1236.



13. Li L, Wang X, Yang B, Wang Y, Gao P, Chen Y, et al. Validation and comparison of drug eluting stent to bare metal stent for restenosis rates following vertebral artery ostium stenting: a single-center real-world study. Interv Neuroradiol 2020;26:629-636.



14. Watanabe H, Domei T, Morimoto T, Natsuaki M, Shiomi H, Toyota T, et al, STOPDAPT-2 Investigators. Effect of 1-month dual antiplatelet therapy followed by clopidogrel vs 12-month dual antiplatelet therapy on cardiovascular and bleeding events in patients receiving PCI: the STOPDAPT-2 randomized clinical trial. JAMA 2019;321:2414-2427.









