 |
 |
- Search
| Neurointervention > Volume 18(3); 2023 > Article |
|
Abstract
Purpose
Materials and Methods
Results
Conclusion
Notes
Ethics Statement
The ethics approval was obtained from the institutional review board and the informed consent was waived. This article does not include any information such as age and sex that may identify the person.
Conflicts of Interest
SHS has been the Editor-in-Chief of the Neurointervention since 2023; however, SHS has not been involved in the peer reviewer selection, evaluation, or decision process of this article. No potential conflict of interest relevant to this article was reported. No other authors have any conflict of interest to disclose.
Author Contributions
Concept and design: SHS. Analysis and interpretation: KCC, TK, and SWH. Data collection: JHK, KCC, and TK. Writing the article: JHK and SHS. Critical revision of the article: KCC, SWH, and SHS. Final approval of the article: SHS. Statistical analysis: JHK and TK. Overall responsibility: SHS.
Fig.┬Ā1.
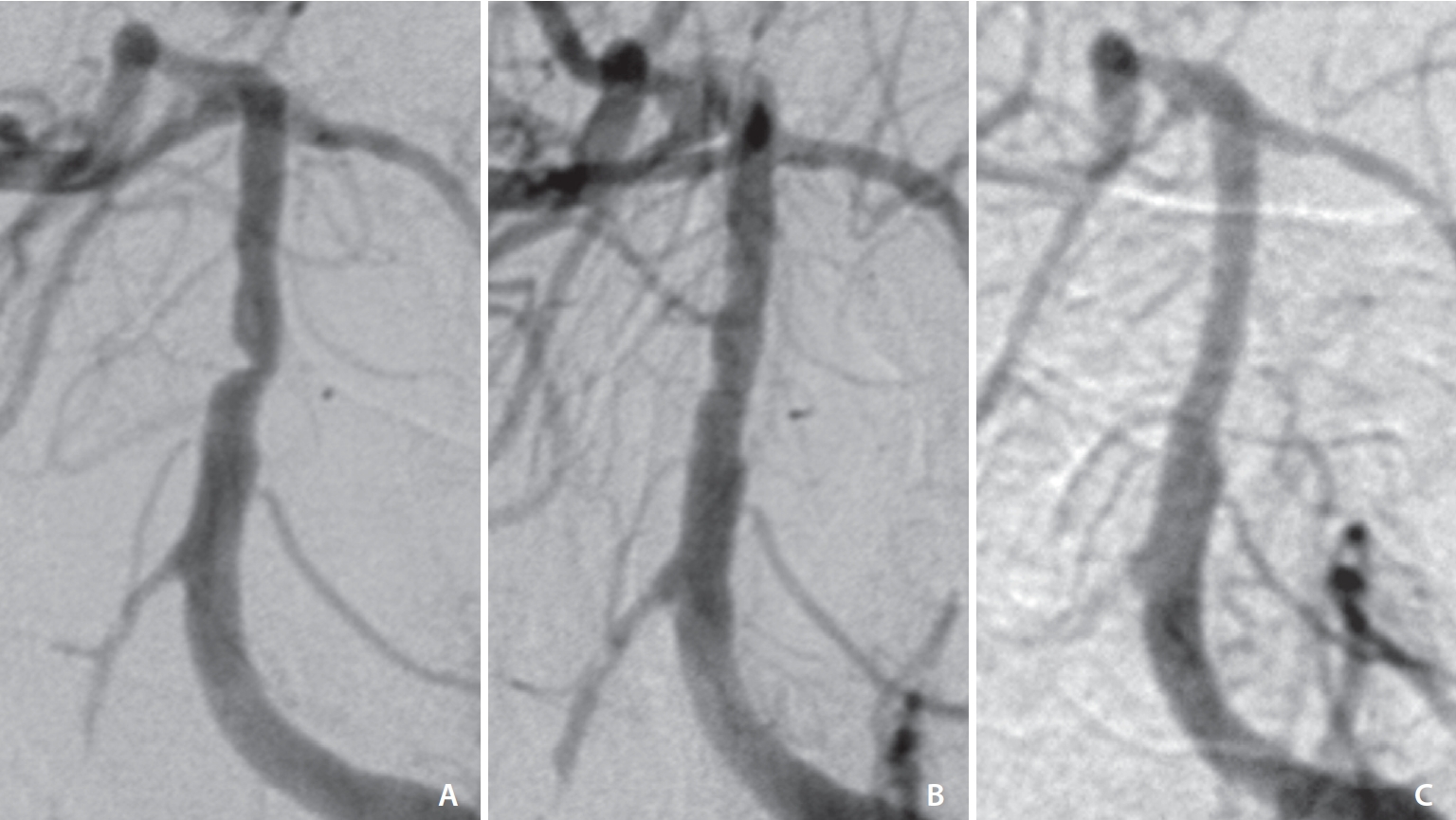
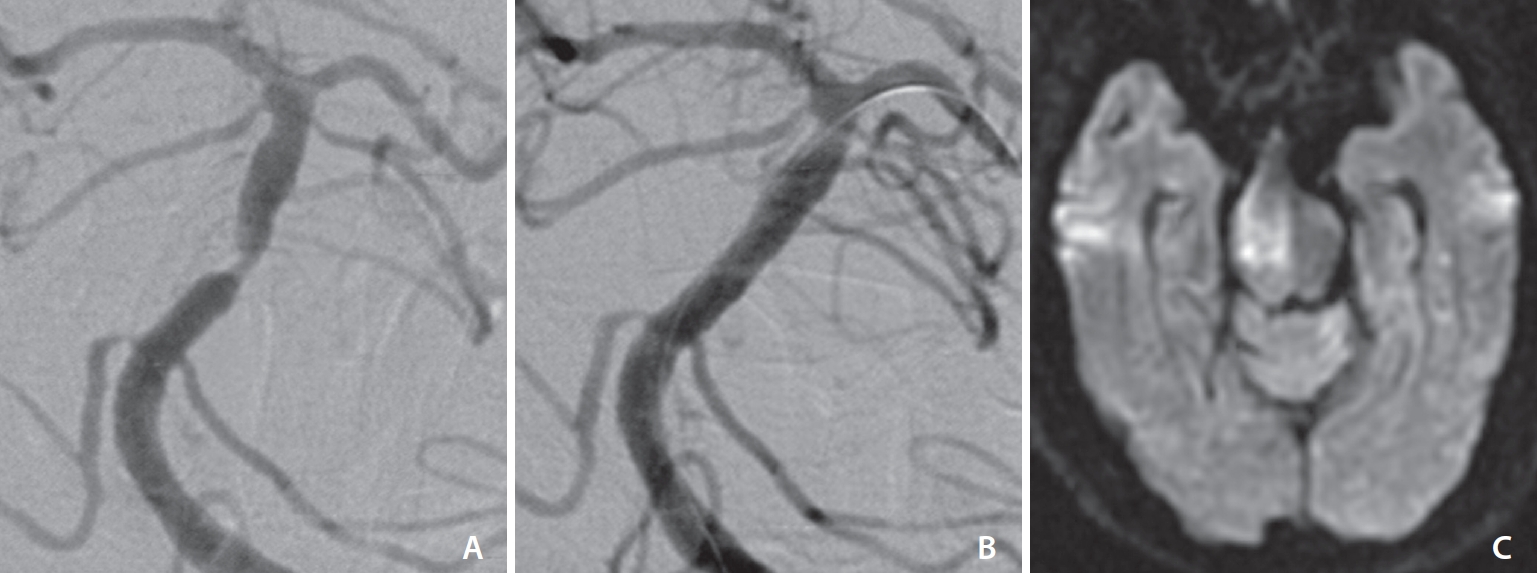
Fig.┬Ā2.
Table┬Ā1.
Table┬Ā2.
REFERENCES
- TOOLS
-
METRICS

-
- 1 Crossref
- 1,497 View
- 215 Download
- Related articles in NI
-
A Review of Endovascular Treatment for Posterior Circulation Strokes2023 June;18(2)
Endovascular Coiling of Fenestrated Vertebrobasilar Cerebral Aneurysms2022 November;17(3)
Y Stent Rendezvous to Treat Symptomatic Innominate Artery Stenosis2022 March;17(1)






