Cutting Balloon Angioplasty for Severe In-Stent Restenosis after Carotid Artery Stenting: Long-Term Outcomes and Review of Literature
Article information
Abstract
Purpose
Cutting balloon-percutaneous transluminal angioplasty (CB-PTA) is a feasible treatment option for in-stent restenosis (ISR) after carotid artery stenting (CAS). However, the longterm durability and safety of CB-PTA for ISR after CAS have not been well established.
Materials and Methods
We retrospectively reviewed medical records of patients with ISR after CAS who had been treated with CB-PTA from 2012 to 2021 in our center. Detailed information of baseline characteristics, periprocedural and long-term outcomes, and follow-up imaging was collected.
Results
During 2012–2021, a total of 301 patients underwent CAS. Of which, CB-PTA was performed on 20 lesions exhibiting severe ISR in 18 patients following CAS. No patient had any history of receiving carotid endarterectomy or radiation therapy. These lesions were located at the cervical segment of the internal carotid artery (n=16), proximal external carotid artery (n=1), and distal common carotid artery (n=1). The median time interval between initial CAS and detection of ISR was 390 days (interquartile range 324–666 days). The follow-up period ranged from 9 months to 9 years with a median value of 21 months. Four patients (22.2%) were symptomatic. The average of stenotic degree before and after the procedure was 79.2% and 34.7%, respectively. Out of the 18 patients receiving CB-PTA, 16 (88.9%) did not require additional stenting, and 16 (88.9%) did not experience recurrent ISR during the follow-up period. Two patients who experienced recurrent ISR were successfully treated with CB-PTA and additional stenting. No periprocedural complication was observed in any case.
Conclusion
Regarding favorable periprocedural and long-term outcomes in our single-center experience, CB-PTA was a feasible and safe option for the treatment of severe ISR after CAS.
INTRODUCTION
In the pivotal randomized trials of carotid artery stenting (CAS), the cumulative risk of severe in-stent restenosis (ISR), which is restenosis of more than 70%, ranged from 3–16.6% at 2–5 years after CAS [1-5]. Although it is debatable whether ISR after CAS increases the risk of stroke occurence [2,5], ISR progression can lead to occlusion of a target artery and potentially predispose patients to ischemic neurologic symptoms [6,7].
The optimal management of ISR following CAS has yet to be established [6]. To date, percutaneous transluminal angioplasty (PTA) remains the most commonly used method for treating ISR after CAS, either with or without additional stenting. Several options are available for angioplasty including plain balloon (PB), cutting balloon (CB), and drug-coated balloon (DCB). Of which, despite the absence of evidence directly comparing the efficacy among different types of balloons, PB-PTA has been the most reported intervention for treating ISR after CAS. However, it has been noted that a better treatment response to the angioplasty and a lower requirement for additional stenting has been observed with CB-PTA compared to PB-PTA for ISR after CAS [8]. Several cases have documented successful treatment of ISR after CAS using CB-PTA without procedural complications, which led to slightly increasing trends in CB utilization since 2005 [8-16].
However, as prior carotid endarterectomy (CEA) or radiation therapy and neck cancer of the ipsilateral carotid artery are known to be associated with a higher risk of ISR after CAS [17,18], a majority of the presented cases of CB-PTA for ISR were obtained from patients having history of undergoing CEA or radiation therapy. Herein, we describe periprocedural and long-term outcomes up to 9 years of 20 cases of CB-PTA in 18 patients with severe ISR after CAS without history of CEA or radiation therapy.
MATERIALS AND METHODS
Study Population and Data Collection
This study was designed as a single-center retrospective observational study. We included every patient who had received CB-PTA for severe ISR after CAS in our center (Seoul National University Bundang Hospital) between July 2012 and September 2021.
We retrospectively reviewed detailed information on baseline characteristics, time interval between initial CAS and detection of ISR, time interval between treatment of ISR and detection of recurrent ISR, degree of calcified plaque, procedural devices used in the initial CAS and treatment of ISR, results of every follow-up imaging, and last clinic visit records. Definition of severe ISR was restenosis of at least 70% of a target artery seen on digital subtraction angiography (DSA) [5]. The grade of stenosis was determined according to the North American Symptomatic Carotid Endarterectomy Trial criteria [19]. The presence of ipsilateral stroke or ischemic neurologic symptoms was defined as symptomatic lesion. Based on the source images of computed tomography angiography (CTA), the degree of calcified plaque was graded as follows: no or minimal amount of calcification, grade 0; small spots of punctate calcification, grade 1; intermediate and non-confluent calcification, grade 2; and large and solid calcified plaque, grade 3 [20].
For the electronic literature search about CB-PTA for ISR until June 2022, we searched PubMed using the following terms: “carotid artery”, “carotid artery stenosis”, “carotid artery stenting”, “instent restenosis”, “instent re-stenosis”, “in-stent restenosis”, “in-stent re-stenosis, “angioplasty”, and “cutting balloon”.
Diagnosis of Severe ISR after CAS
The initial CAS procedure was done according to recommended CAS protocols in Korea [21]. Patients treated with CAS underwent regular follow-up screening for ISR using duplex ultrasonography (DUS), CTA, or magnetic resonance angiography (MRA). Once severe ISR was suspected on a screening exam, DSA was conducted to confirm the diagnosis of ISR. When reaching a decision that the benefit of reintervention outweighed the procedural risk, we performed CB-PTA with or without additional stenting for severe ISR.
Medical Treatment
Every subject in this study had been taking either single or dual antiplatelet medication at the time of diagnosis with severe ISR after CAS. Once severe ISR was suspected in a screening exam, patients taking single antiplatelets were switched to dual antiplatelet therapy with aspirin and clopidogrel, unless they had a history of intolerable bleeding or allergic complications. For patients who were already taking dual antiplatelet therapy, a decision to change the combination of medications was left to the discretion of the treating physicians.
Interventional Techniques and Follow-up Protocols
We used a 6F guiding sheath or a 9F balloon-guide catheter via the common femoral artery under local anesthesia. A total dose of 2,000- to 4,000-unit of unfractionated heparin was given intravenously. To navigate the distal part of the target lesion, a 0.014-inch microwire was used. Proximal or distal embolic protection devices were routinely used to prevent migration of emboli. Then, we performed CB-PTA at the site of ISR. A 4×15 mm Small Peripheral CB (Boston Scientific) was inflated slowly to a pressure ranging from 6 to 12 atmospheres, ensuring sufficient dilation within the stenotic lesion was attained. If it was unable to place the CB at the target lesion, PTA with a 2-mm PB was performed before CBPTA. If residual stenosis of more than 50% remained after CBPTA, additional PTA with a larger PB was implemented. We conducted additional stent placement in case of immediate recoil or recurrent ISR after PTA.
Follow-up for recurrent ISR was screened after PTA with or without additional stenting using DUS, CTA, or MRA at 1 month, 6 months, 1 year, and every 1–2 years thereafter.
RESULTS
Baseline Characteristics
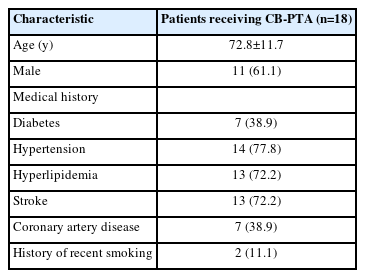
During the study period of 2012–2021, a total of 301 patients underwent CAS in our center. Of which, 18 patients with severe ISR after CAS who underwent CB-PTA were included in this study (Table 1). The mean age was 72.8±11.7 years, and 11 patients (61.1%) were male. Regarding the medical history, 7 patients (38.9%) had diabetes, 14 (77.8%) had hypertension, 13 (72.2%) had hyperlipidemia, 13 (72.2%) had stroke, 7 (38.9%) had coronary artery disease, and 2 (11.1%) had a recent smoking history. Of the 18 patients, 17 (94.4%) had 1 or more vascular risk factors. None of the patients had a history of receiving CEA or radiation therapy.
Periprocedural and Long-term Outcomes
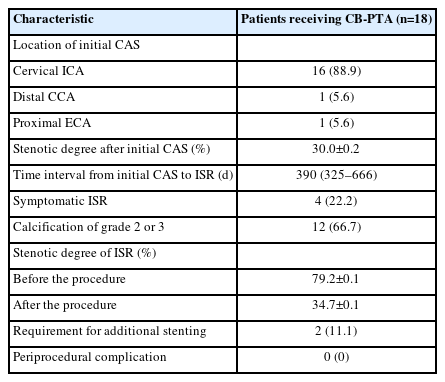
Among the 18 patients included in this study, 16 patients (88.9%) received initial CAS for the cervical ICA, 1 patient received CAS for the common carotid artery, and 1 patient received CAS for the external carotid artery (Table 2). The median (interquartile range) time interval between initial CAS and detection of ISR was 390 days (324–666 days). Of these patients, 4 patients (22.2%) were symptomatic, and 12 patients (66.7%) had calcification of grade 2 or 3. The mean stenotic degree before and after CB-PTA was 79.2% and 34.7%, respectively. Two patients (11.1%) required additional stenting after CB-PTA.
During the follow-up period, which ranged from 9 months to 110 months (median 21 months), 2 patients (11.1%) who underwent CB-PTA for severe ISR experienced recurrent severe ISR at 720 and 462 days, respectively (Table 3). Both patients were asymptomatic and successfully treated with CB-PTA and additional stenting. One patient was followed up for 50 months after the treatment, during which no recurrent ISR was found on the follow-up screening exam.
No periprocedural complication was observed in any of the patients receiving CB-PTA in this study.
Review of Literature
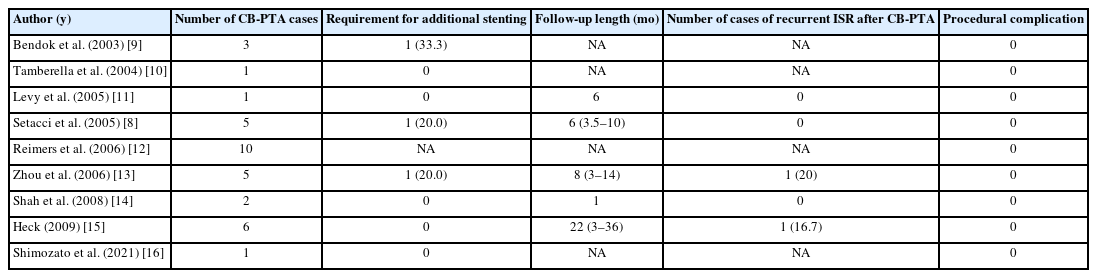
According to our comprehensive literature review, a total of 34 cases of ISR involving successful treatment using CB-PTA have been documented in 9 case reports (Table 4) [8-16]. Following the initial report by Bendok et al. [9] in 2003, reporting 3 cases of CB-PTA for ISR, a number of subsequent case reports have been published. Follow-up data after the procedure was available in 5 of the identified case reports. Levy et al. [11] reported a single case of CB-PTA and conducted follow-up for 6 months. Setacci et al. [8] presented 5 cases of CB-PTA with an average follow-up period of 6 months (ranging from 3.5–10 months). In a study by Zhou et al. [13], 5 cases of ISR treated with CB-PTA were followed up for an average of 8 months (ranging from 3–14 months). Of which, 1 case (20%) of recurrent ISR was observed and successfully treated with CB-PTA. Heck [15] reported 6 cases of CB-PTA with a maximum follow-up duration of 36 months. During the follow-up period, 1 case (16.7%) of recurrent ISR after CB-PTA was noted, which did not require additional treatment.
Out of 24 cases with available procedural data, 3 cases (12.5%) required additional stenting to manage residual stenosis following CB-PTA for ISR. Among 19 cases with available follow-up data, 2 cases (10.5%) of recurrent ISR after CBPTA were reported at 8 months and 20 months following CBPTA, respectively.
DISCUSSION
In this study, we reviewed 18 patients suffering from severe ISR after CAS who were successfully treated with CB-PTA. Only 2 of these patients (11.1%) necessitated additional stenting, and 2 (11.1%) encountered recurrent ISR following the CB-PTA treatment during a median follow-up of 21 months (ranging from 9 to 110 months). It is noteworthy that no procedural complication was observed in a total of 54 cases that includes our study and the previous literature. To the best of our knowledge, this study represents the largest available data on CB-PTA for severe ISR after CAS with the longest follow-up period.
Due to the absence of treatment guidelines for ISR after CAS, the selection of treatment options varies depending on the treating physicians and centers [6]. Endovascular approaches, such as balloon angioplasty with various types of balloons (PB, CB, or DCB), or additional stenting, as well as surgical interventions including CEA with stent removal, carotid artery bypass, or interposition graft, can be considered as viable options for ISR. There is a lack of comparative studies between variable treatment options for ISR after CAS. Additionally, there is insufficient evidence to establish the superiority of CB-PTA over PB-PTA in patients with ISR of the carotid arteries. However, prior studies on coronary interventions have suggested potential advantages of CBPTA over PB-PTA in the treatment of ISR [22,23]. First, CB-PTA requires fewer attempts of balloon angioplasty than PB-PTA. Second, the rate of additional stent placement tends to be lower in CB-PTA compared to PB-PTA. Third, CB-PTA shows a lower frequency of balloon slippage due to microblades (atherotomes) anchoring the balloon to the plaque during inflation, and subsequently reduces the risk of arterial dissection near the stent margins. Fourth, the microblades on the surface of the CB can surgically incise the neointimal tissues or atherosclerotic plaque within the stent and extrude fragmented tissues or plaque out of the stent [24,25]. Therefore, a CB can easily achieve a larger acute gain in luminal diameter compared to a PB while posing a low risk of stent overexpansion. In our study, most of the patients (66.7%) had highgrade calcification.
Despite several advantages of a CB over PTA, it is important to note that CB-PTA carries the risk of vessel injury or arterial perforation due to the microblades. Nevertheless, it has been proposed that CB-PTA can be safely performed for ISR after CAS, as the implanted stent acts as a protective barrier against potential microblades-induced vessel wall injury [26]. Hence, a CB could be safely inflated up to the stent diameter. Furthermore, a small-sized CB was employed to minimize the potential risk of vessel injury outside the stent.
Among the 5 previous studies which provided follow-up data of CB-PTA for ISR, as shown in Table 4, the rate of requirement for additional stenting was 12.5% (3/24), and the incidence of recurrent ISR after CB-PTA for ISR was 10.5% (2/19). These findings closely align with the results of our study. However, it is to be noted that the previous studies did not encompass patients with late ISR beyond 2 years after CAS and without history of receiving CEA or radiation therapy. Regarding the mechanism underlying ISR after CAS, neointimal hyperplasia is known to play a crucial role in the earlier period (within 2 years after CAS), while atherosclerosis may reemerge in the later period (beyond 2 years after CAS) [27]. Of the 18 patients in our study, 5 patients were diagnosed with severe ISR beyond 2 years after initial CAS, and no patient had any history of receiving CEA or radiotherapy. It could be suggested that CB-PTA might be safely performed not only for neointimal hyperplasia but for atherosclerotic plaque within the stent in patients with ISR after CAS, which aligns with the evidence from studies on coronary interventions of ISR.
Given the low incidence of ISR and the absence of well-designed registries of patients with ISR, this study has significant value in collecting relatively large data about the long-term safety and durability of CB-PTA for ISR after CAS. Furthermore, unlike previous studies that employed different criteria for determining the degree of stenosis in ISR using various screening modalities, we confirmed the diagnosis of severe ISR by DSA. As a limitation, due to the retrospective nature of this study at a single center and the absence of control arms, it was not possible to establish the superiority of CB-PTA for carotid ISR over PB-PTA. Additionally, a comparison with other treatment modalities for ISR was unavailable in this study.
CONCLUSION
CB-PTA can be considered as a feasible and safe treatment option for severe ISR following CAS. Further well-designed comparative studies or randomized trials are needed to establish the efficacy of CB-PTA.
Notes
Fund
None.
Ethics Statement
This study was approved by the Institutional Review Board at Seoul National University Bundang Hospital (IRB No. B-2401-878-103).
Considering the absence of health insurance coverage for CB in carotid ISR in South Korea, explicit consent was obtained from each patient before the procedure. Each patient was informed that CB has been extensively utilized in cases of peripheral artery and coronary ISR with health insurance coverage.
The consent for publication is not required as the submission does not include any images for information that may identify the person.
Conflicts of Interest
The authors have no conflicts to disclose.
Author Contributions
Concept and design: JYL, Jonguk Kim, DYK, JYK, SHB, Jihoon Kang, BJK, HJB, and CJ. Analysis and interpretation: JYL, Jonguk Kim, and CJ. Data collection: JYL, MSK, Jonguk Kim, DYK, JYK, SHB, Jihoon Kang, BJK, HJB, and CJ. Writing the article: JYL and CJ. Critical revision of the article: JYL, Jonguk Kim, JYK, SHB, and CJ. Final approval of the article: JYL and CJ. Statistical analysis: JYL and CJ. Overall responsibility: JYL and CJ.