Findings of Angiography and Carotid Vessel Wall Imaging Associated with Post-Procedural Clinical Events after Carotid Artery Stenting
Article information
Abstract
Purpose
Vessel wall imaging (VWI) for carotid plaque is better for detecting unstable carotid plaque such as intraplaque hemorrhage (IPH), lipid-rich necrotic core (LRNC), and thin/ruptured fibrous cap. However, the role of VWI before carotid artery stenting (CAS) is unclear. Thus, this study aimed to determine the findings of symptomatic carotid stenosis before CAS on angiography and carotid VWI and to evaluate the imaging findings associated with post-procedural clinical events after CAS.
Materials and Methods
This retrospective study included 173 consecutive patients who underwent carotid VWI, CAS, and post-procedural diffusion-weighted imaging (DWI) after CAS. Findings of unstable plaque on carotid VWI and unstable findings on angiography were analyzed. We also analyzed the incidence of post-procedural clinical events, any stroke, myocardial infarction (MI), and death within 30 days of CAS.
Results
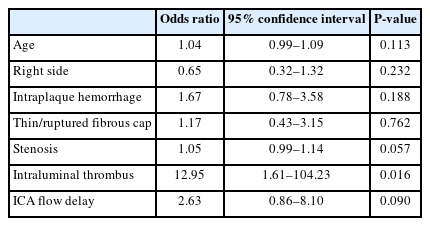
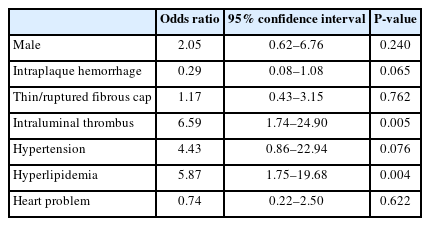
Of 173 patients, 101 (58.4%) had initial ischemic symptoms and positive findings on DWI. Symptomatic patients were significantly higher in patients with IPH than in patients without IPH (62.4% vs. 45.8%, P=0.031). Degree of stenosis, thrombus of the stenotic lesion, flow delay of internal carotid artery, and flow arrest by filter thrombus had significantly higher prevalence in the symptomatic group. Twenty patients (11.6%) had post-procedural clinical events such as any stroke, clinical symptoms, and/or MI. Hyperlipidemia and intraluminal thrombus on angiography were identified as significant factors influencing post-procedural events after CAS.
Conclusion
An intraluminal thrombus on angiography was identified as a significant factor influencing post-procedural clinical events after CAS.
INTRODUCTION
Extracranial carotid artery stenosis is a significant cause of stroke [1]. Carotid revascularization has 2 options: carotid endarterectomy (CEA) and carotid artery stenting (CAS). CEA has been the gold standard treatment for carotid artery stenosis. CAS is an appealing, less invasive alternative to CEA. A randomized trial has shown that CAS and CEA have similar short- and long-term outcomes [2]. Other studies have suggested that CAS using an embolic-protection device is not inferior to CEA [3].
Recently, vessel wall imaging (VWI) of carotid plaque is better for detecting unstable carotid plaque such as intraplaque hemorrhage (IPH), lipid-rich necrotic core (LRNC), and thin/ruptured fibrous cap. Especially, carotid IPHs found in preoperative images are significantly associated with new emboli after CAS [4]. Recently, it has been shown that massive IPHs are associated with ipsilateral new ischemic lesions after CAS [5]. However, another study has reported no significant difference in primary outcome between IPH and non-IPH groups after CAS [6]. Therefore, the role of carotid VWI before CAS remains unclear. During CAS, cerebral angiography can detect the degree of stenosis, thrombus of the stenotic lesion, ulceration, and collateral status. Ulceration is associated with an increased embolic risk during CAS [7]. A longer carotid plaque predicts adverse outcomes after CAS [8]. However, no studies have reported the correlation between post-procedural clinical events and unstable findings on carotid VWI or carotid angiography after CAS. Therefore, this study aimed to determine the findings of symptomatic carotid stenosis before CAS on angiography and carotid VWI and to evaluate the imaging findings associated with post-procedural clinical events after CAS.
MATERIALS AND METHODS
Patients
This retrospective study assessed 213 consecutive patients who underwent CAS because of symptomatic or asymptomatic carotid stenosis between January 2018 and October 2022. All patients had symptomatic carotid artery stenosis of more than 50% (North American Symptomatic Carotid Endarterectomy Trial [NASCET] criteria) or asymptomatic carotid artery stenosis of more than 70% (NASCET criteria). Carotid stenosis in symptomatic patients was initially detected by a stroke magnetic resonance (MR) or computed tomography protocol. Stenosis of asymptomatic patients was diagnosed by carotid sonography and/or a routine brain MR imaging (MRI) that included contrast-enhanced MR angiography to evaluate neurologic symptoms. Multicontrast carotid plaque MR was performed within 1 week before CAS. New postoperative ischemic lesions were assessed by diffusion-weighted imaging (DWI) within 24 hours after CAS. Of these patients, those with poor imaging quality of carotid plaque MR or DWI after CAS caused by pulsation or motion artifacts or additional stenotic lesions in the territorial intracranial artery (>50%; NASCET criteria) were excluded.
We reviewed patients’ cardiovascular risk factors, including hypertension, diabetes mellitus, hyperlipidemia, and previous clinical history of stroke and heart problems. Detailed guidelines for hyperlipidemia were as follows: we diagnosed patients with hyperlipidemia when patients had already been diagnosed with hyperlipidemia and had been taking statin drugs or when the results of the lipid profile, which was determined at the time of admission, met 1 of the following criteria: 1) triglyceride >200 mg/dL, 2) high-density lipoprotein cholesterol <40 mg/dL, and 3) low-density lipoprotein cholesterol satisfying the criteria specified in the 2018 Guideline on the Management of Blood Cholesterol published by the American College of Cardiology [9]. Previous heart problems were defined as an existing diagnosis of atrial fibrillation/atrial flutter and a history of coronary heart disease or myocardial infarction (MI).
Carotid Plaque MRI
Carotid plaque MR examinations were performed on a 3.0 T scanner (Achieva; Philips Medical Systems) with a 16-channel head coil. Carotid MR protocol for evaluating atherosclerotic components included the following 5 different scans: timeof-fight (TOF) MR angiography, black-blood (BB) T1-weighted, BB T2-weighted, BB post-contrast T1-weighted, and simultaneous non-contrast angiography and intraplaque hemorrhage (SNAP). The center of all sequences was at the bifurcation of the index artery with the carotid plaque. BB T1-weighted, BB T2-weighted, and BB post-contrast T1-weighted sequences were obtained with a slice thickness of 2.0 mm without interslice spacing. TOF axial imaging and SNAP imaging used a slice thickness of 1.0-mm without interslice spacing. Images were obtained with a 14×14 cm field of view and a matrix size of 216×192. Total acquisition time was approximately 30 minutes.
CAS Procedure
All procedures were performed by 1 interventional neuroradiologist with 20 years of experience under local anesthesia via a percutaneous transfemoral route. Systemic anticoagulation was initiated with a 3,000-U bolus of IV heparin, followed by a 1,000-U/h infusion. A double coaxial system was placed in the common carotid artery (CCA) to enable stent placement. If an intraluminal thrombus was seen on initial carotid angiography, aspiration thrombectomy was performed to prevent thrombus migration before a protection device was inserted. CAS was performed using an Emboshield distal embolic protection system (Abbott Vascular). Pre-dilatation was performed with a 3–5 mm balloon catheter in consideration of the degree of stenosis, presence of symptoms, or status of collateral flows. After balloon dilatation, a self-expandable stent (RX Acculinx) was deployed. The size was chosen according to the presumed parent size. Post-stenting angioplasty was performed in patients with residual diameter stenosis >20%. All patients were monitored in the intensive care unit for 24 hours after the procedure. DWI was performed within 24 hours after CAS.
Definition and Outcomes
Carotid plaque MRI and carotid angiography were reviewed retrospectively by 2 neuroradiologists with 25 years and 15 years of experience, respectively, who were blinded to the clinical information of each patient and the purpose of this study. When their interpretations differed, consensus interpretation was used for the final analysis.
Poor imaging quality of carotid plaque MRI was defined as ill-defined visualization of the inner and outer walls of the carotid artery due to motion artifact or pulsation. Carotid plaque MRI was analyzed to determine IPH, LRNC, and the status of the fibrous cap (Fig. 1). IPH was defined as the presence of a hyperintense signal within plaque greater than 200% of the signal intensity of the adjacent muscle for at least 2 consecutive slices on SNAP imaging [10]. LRNC was defined as a relatively lower signal than adjacent fibrous tissue on BB post-contrast T1-weighted sequence due to reduction or absence of enhancement [11]. The intact fibrous cap was defined as a relatively greater enhancement than the underlying LRNC on BB post-contrast T1-weighted sequence [11]. A thin/ruptured fibrous cap was defined as a non-enhanced fibrous cap [11].

Imaging findings of carotid plaque magnetic resonance imaging (MRI). (A) Intraplaque hemorrhage (arrows). (B) Lipid-rich necrotic core (LRNC) and thin/ruptured fibrous cap. LRNC: non-enhanced area in the plaque on contrast-enhanced MRI (arrow). Thin/ruptured fibrous cap: non-enhanced fibrous cap. Vessel lumen (asterisk).
Imaging analysis of carotid angiography for CAS was performed to determine the degree of stenosis (NASCET criteria), ulcer, intraluminal thrombus of stenotic lesions, internal carotid artery (ICA) flow delay compared to external carotid artery (ECA), and ICA flow arrest by filter thrombus during CAS (Fig. 2). Thrombectomy was performed before CAS in patients with intraluminal thrombus to reduce embolic risk. Symptomatic carotid artery stenosis was defined as focal neurologic symptoms with DWI-positive imaging occurring within 1 week of CAS attributable to an ipsilateral carotid artery vascular distribution [12]. Neurologic evaluation was performed at baseline, immediately after the procedure, 24 hours after the procedure, at the time of any change in clinical symptoms, before patient discharge, and 1 month after discharge by a stroke neurologist.
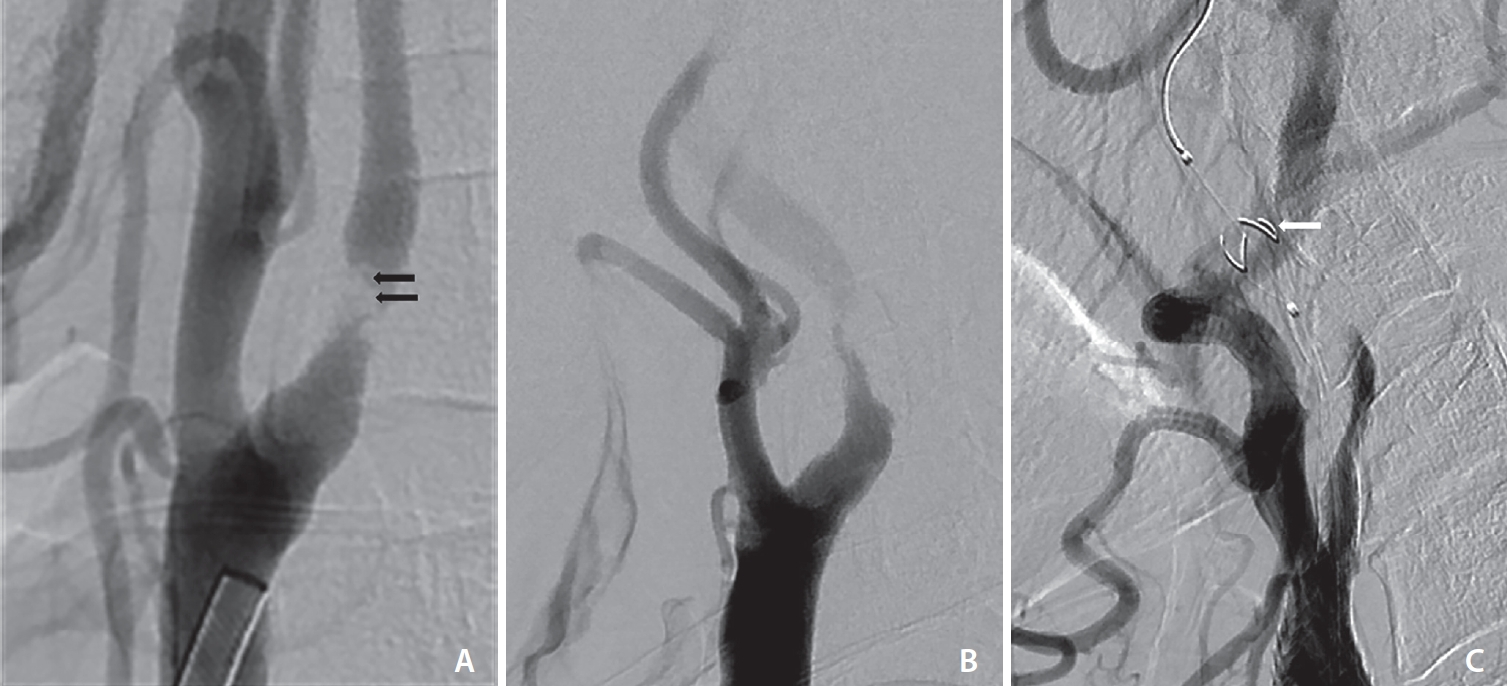
Imaging findings of carotid angiography. (A) Intraluminal thrombus of the stenotic lesion (arrows). (B) Internal carotid artery (ICA) flow delay compared to external carotid artery. (C) ICA flow arrest by filter thrombus during carotid artery stenting (arrow).
Predictors of clinical outcomes after CAS were analyzed: the incidence of post-procedure clinical events with symptoms such as headache, nausea, vomiting, any stroke, MI, and death within 30 days after CAS. A minor stroke was defined as evidence of neurologic deterioration based on a <4-point increase in National Institutes of Health Stroke Scale (NIHSS) without the presence of aphasia or hemianopsia or complete recovery within 1 month. A major stroke was defined as a ≥4-point increase in NIHSS, the presence of aphasia or hemianopsia, or any residual deficit beyond 1 month. MI was defined as the combination of elevated cardiac enzymes to a value of 2 or more times the upper limit of normal plus chest pain.
Statistical Analysis
All statistical analyses were performed with SPSS for Windows version 24.0 (IBM Co.). Continuous variables were presented as mean±standard deviation or mean±interquartile range (IQR). They were analyzed using an independent t-test. Categorical variables were presented as numbers and percentages. They were analyzed using the χ2 test or Fisher exact test. Univariate analysis was performed to explore possible risk factors for neurological symptoms after CAS. Statistical significance in the univariate analysis was set as P<0.10. Multivariate logistic regression analysis was then performed to determine the independent association between complications after CAS and imaging findings. Statistical significance was defined as P<0.05.
RESULTS
Patients
Of 213 consecutive patients who underwent carotid plaque MRI before CAS procedure and DWI within 24 hours after CAS, 17 were excluded because of poor imaging quality of carotid VWI and 23 were excluded because of additional stenotic lesion in the territorial intracranial artery (cavernous or petrous ICA: 8; middle cerebral artery: 15). Finally, 173 patients (median age, 75 years [IQR, 70–80 years]; male, n=135 [78.0%]) were enrolled in this study. Demographic data of the symptomatic group and the asymptomatic group in this study are shown in Table 1. A total of 101 (58.4%) patients showed acute symptoms with positive findings on DWI. The symptomatic group appeared to have a significantly higher in the right carotid artery than the asymptomatic group (59.4% vs. 41.7%, P=0.021).
Imaging Analysis between Symptomatic and Asymptomatic Groups
Imaging findings of carotid plaque MR and carotid angiography of the 2 groups are shown in Table 2. On carotid plaque MRI, the percentage of patients with IPH was significantly higher than that of patients without IPH in the symptomatic group (62.4% vs. 45.8%, P=0.031). On carotid angiography during CAS, the degree of stenosis was significantly higher in the symptomatic group than in the asymptomatic group (78.43±0.84 vs. 75.52±0.67, P=0.007), ICA flow delay compared to ECA due to high-grade stenosis was significantly higher in the symptomatic group (22.8% vs. 8.3%, P=0.012). Intraluminal thrombus of stenotic lesion on initial carotid angiography was significantly higher in the symptomatic group (27.7% vs. 1.4%, P<0.001). Flow arrest of ICA by filter thrombus also had a significantly higher prevalence in the symptomatic group (15.8% vs. 2.8%, P=0.006).

Imaging analysis of carotid plaque MR and carotid angiography for symptomatic and asymptomatic groups
Multivariate analysis was performed for factors associated with symptomatic carotid plaques (Table 3). Results confirmed that the intraluminal thrombus was a significant risk factor associated with symptomatic carotid plaques (odds ratio [OR], 12.95 [1.61–104.23]; P=0.016).
Post-procedural Clinical Outcomes after CAS
Twenty (11.6%) patients had the post-procedural clinical events after CAS. Of these patients, 6 (3.4%) appeared to have major stroke (dysarthria, n=2; motor disorder, n=2; dysarthria and motor disorder, n=2), 7 (4.0%) appeared to have minor stroke (mild dysarthria, n=4; mild motor disorder, n=3), and 6 had clinical symptoms without stroke. Also, 1 patient had MI 10 days after CAS. He was discharged at home after coronary stenting. Clinical symptoms after CAS showed nausea (n=8), vomiting (n=8), and/or headache (n=6). Any procedure related to hemorrhage after CAS did not appear during the study period.
Demographic data and imaging analysis associated with post-procedural clinical events after CAS are shown in Table 4. Hyperlipidemia and hypertension had a significantly higher prevalence in those with post-procedural clinical events (P<0.05). However, LRNC had a significantly higher prevalence in those without post-procedural clinical events (P=0.007).
Multivariate analysis was performed for factors associated with post-procedural clinical events after CAS (Table 5). Hyperlipidemia was identified as an influencing factor for post-procedural events after CAS (OR, 5.87 [1.75–19.68]; P=0.004). Intraluminal thrombus on carotid initial angiography was also significantly associated with post-procedural clinical events after CAS (OR, 6.59 [1.74–24.90]; P=0.005).
DISCUSSION
In this study, intraluminal thrombus on carotid angiography and hyperlipidemia were identified as risk factors for post-procedural events after CAS. However, unstable plaque findings on carotid VWI were not associated with peri-procedural events. In multivariate analysis, intraluminal thrombus on carotid angiography was associated with peri-procedural events.
Carotid VWI can be used to assess carotid plaque burden and components [13,14]. Unstable plaque findings on carotid VWI are associated with a higher risk of ischemic stroke. They include IPH, a LRNC, and a thin or ruptured fibrous cap [11,14]. A meta-analysis [14] reported that the incidence of stroke is statistically significantly different between IPH plaques and LRNC plaques (relative risk [RR], 1.27; 95% confidence interval [CI], 1.04–1.55; P<0.05) and between IPH plaques and calcification plaques (RR, 2.99; 95% CI, 1.74–5.14; P<0.0001). Furthermore, there was a statistically significant difference between thinned or ruptured fibrous caps plaques and carotid artery stenosis (RR, 10.84; 95% CI, 5.60–20.98; P<0.0001) and between calcification plaques and carotid artery stenosis (RR, 0.83; 95% CI, 0.75–0.92; P<0.0001). Therefore, IPH and thinned or ruptured fibrous caps might play an important role in predicting stroke. IPH is also strongly associated with recurrent ischemic events. It could predict recurrent ipsilateral stroke [6,15,16]. In our study, we analyzed imaging data considering all 3 characteristics of unstable plaques on carotid VWI and found that the percentage of patients with IPH was significantly higher than that of patients without IPH in the symptomatic group. This finding suggests that the measurement of luminal stenosis alone might not be sufficient for the source of stroke [17]. Carotid VWI might be necessary for evaluating plaque components. MR plaque imaging not only predicts the risk of stroke recurrence but also can help optimize treatment selection for individual patients by differentiating between unstable/vulnerable and stable plaques [18].
In our study, high-grade stenosis, intraluminal thrombus, ICA flow delay, and flow arrest by the filter thrombus during CAS were significantly higher in the symptomatic group. Intraluminal thrombus was especially associated with symptomatic carotid plaques in the multivariate analysis. Intraluminal thrombus of the carotid stenotic lesion is a precursor to potential tandem occlusion [19]. Approximately 15% of patients undergoing endovascular thrombectomy for anterior circulation stroke have a tandem lesion defined as severe stenosis or occlusion of the cervical ICA ipsilateral to its intracranial occlusion [20]. Intraluminal thrombus is most often atherosclerotic in nature, with an underlying plaque becoming unstable, rupturing, and triggering local thrombus formation [19]. Therefore, thrombectomy should be performed before CAS in patients with intraluminal thrombus to reduce the embolic risk that may occur during CAS [21,22].
Previous studies have investigated the impact of carotid IPH on patients after CAS [6,12,23]. However, the results of these studies have been conflicting. Yoshimura et al. [23] reported that ischemic events after CAS are more frequent in the IPH-positive group and that the presence of IPH in the plaque is the only independent predictor of postoperative ischemic symptoms. However, some studies have reported that IPH is not a significant risk factor for cerebral embolism during protected carotid artery stent placement in patients with severe carotid stenosis [6,12]. Plaque ulceration is not a significant risk factor for cerebral embolism after CAS [24]. In our study, risk factors of post-procedural events after CAS were analyzed, including unstable findings (such as IPH, LRNC, thin or ruptured fibrous cap on carotid VWI), intraluminal thrombus, degree of stenosis, ICA flow delay, and flow arrest during CAS on carotid angiography. Our results correlated with those of studies suggesting that unstable plaque findings on carotid VWI were not associated with post-procedural clinical events. Although patients with IPH had more symptoms compared to those without IPH, we did not find any significant differences in primary outcomes after stenting, regardless of unstable plaques.
Clinical and morphological parameters predictive for cerebral embolization during CS have been studied previously. Age, hypertension, lesion length, lesion eccentricity, lesion ulceration, and aortic arch type III have been reported to have significant associations with new ischemic lesions during CAS [25-27]. Angiographic factors including left carotid artery intervention, stenosis >90%, ulcerated and calcified plaques, lesions length >10 mm, thrombus at the site, ostial involvement, pre-dilatation without protection device, ICA-CCA angulation >60%, aortic arch type III, and aortic arch calcification were associated with 1 month stroke and/or death [28]. However, another study has found no significant associations of adverse outcomes after CAS with ulceration, degree of stenosis, or presence of contralateral occlusion [28,29]. The risk of perioperative stroke and death after CEA is associated with intraluminal thrombus [30]. In our study, intraluminal thrombus, among factors of carotid angiography, was found to be a significant risk factor for peri-procedural clinical events in the symptomatic group. Although initial thrombectomy was performed for patients with intraluminal thrombus, some residual thrombus could be the embolic source during CAS.
Hyperlipidemia and hypertension have been identified as risk factors for stroke [31,32]. Furthermore, there have been studies identifying risk factors for peri-procedural clinical events, specifically stroke, after CAS. One study has reported that hypertension is a risk factor for procedural-related cerebral embolic lesions during embolic-protected CAS [25]. In another study, hyperlipidemia was identified as a risk factor for death/stroke after CAS on a multivariate analysis using logistic regression [33]. The present study also aimed to identify risk factors associated with CAS-related stroke using demographic data of patients and found that hyperlipidemia was a risk factor for post-procedural clinical events after CAS in multivariate analysis.
This study has several limitations. First, this study had a retrospective design. However, patients who underwent imaging studies and CAS procedures with the same protocol were included. Second, we categorized symptomatic patients using DWI. The results of further research might be different from those of our study if the definition of symptomatic carotid artery stenosis changes. Third, the number of patients enrolled was too small to evaluate the association with the incidence of stroke.
CONCLUSION
Hyperlipidemia and intraluminal thrombus are risk factors for peri-procedural events after CAS. However, unstable plaque on carotid VWI was not a significant risk factor for stroke, MI, or death after protected CAS. Protected CAS might be feasible and safe despite the presence of unstable plaque findings on carotid VWI. Further work and clinical trials are necessary to determine whether unstable plaques on carotid VWI could reflect the risk of post-procedural complications after CAS. In patients with intraluminal thrombus on carotid angiography, a proximal occlusion protection device can be an alternative option for reducing embolic complications after CAS.
Notes
Fund
This paper was supported by the Fund of Biomedical Research Institute, Jeonbuk National University Hospital and by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2023R1A2C1004093).
Ethics Statement
The retrospective study was performed according to the protocol (JUH 2022-10-09) approved by institutional ethics committee of Jeonbuk National University Hospital and informed consent was waived due to the retrospective nature of this study. The consent for publication is not required as the submission does not include any images or information that may identify the person.
Conflicts of Interest
The authors have no conflicts to disclose.
Author Contributions
Concept and design: HSK. Analysis and interpretation: SJ, HP, HSK, and SBH. Data collection: SJ and HP. Writing the article: SJ and HP. Critical revision of the article: SBH. Final approval of the article: HSK and SBH. Obtained funding: HSK. Overall responsibility: HSK.