A “Radial Ready” Tricoaxial Setup for Anterior Circulation Mechanical Thrombectomy: Technical Aspects and Preliminary Results
Article information
Abstract
Purpose
Mechanical thrombectomy (MT) is the standard of care for acute ischemic stroke (AIS) due to large vessel occlusion (LVO). The choice of a transradial approach (TRA) for anterior circulation LVOs is still debatable; the use of a specific tricoaxial system could help mitigate numerous issues related to transradial MT.
Materials and Methods
From November 2022 to November 2023, 22 patients underwent TRA-MT for anterior circulation LVOs, both as first-line and rescue from transfemoral approach (TFA) failure, with the same triaxial setup consisting of a 7F introducer sheath, 7F guide catheter, and aspiration catheters ranging from 5.5F to 5F in relation to the occlusion site. Choice of thrombectomy technique was at operator discretion. Patients’ demographic data, clinical presentation, treatment details, complications, rate of crossover to TFA, successful revascularization (modified thrombolysis in cerebral infarction [mTICI] score ≥2b), and good clinical outcome at 3 months (modified Rankin scale [mRS] 0-2) were reported.
Results
Of 20 patients selected, 10 (50%) had occlusion of M1 segment of middle cerebral artery (MCA), 6 (30%) of internal carotid artery (ICA) terminus, and 4 (20%) with M2 MCA occlusions; 12/20 (60%) were right-sided occlusions and 8/20 (40%) were left-sided. The mean National Institutes of Health Stroke Scale score was 9.25 at admission. Successful revascularization to mTICI 2b-3 was achieved in 18/20 patients (90%). Intracranial complications were reported in 2 (10%) patients. Rate of radial artery occlusion at 24 hours was 10,6%; no access-site haemorrhagic complications were reported. Symptomatic intracranial hemorrhage occurred in 2 (10%) patients. mRS score 0-2 at 3 months was 50%.
Conclusion
The high technical effectiveness and good safety profile of this specific tricoaxial setup for TRA-MT in AIS, even for large proximal LVOs, could constitute a viable alternative to TFA-MT in selected cases.
INTRODUCTION
In recent years, transradial approach (TRA) for neurovascular procedures has gained widespread consideration mainly because of much fewer access site adverse events in comparison with the transfemoral approach (TFA) [1]; regarding mechanical thrombectomy (MT), however, the choice of TRA approach, especially for anterior circulation occlusions, is still under debate, given the need of a specific learning curve in a setting of a time-sensitive procedure. In complex epiaortic vessel anatomy (e.g., bovine arch, type III aortic arch, extracranial tortuosity/kinking/loop), TRA catheterization is expected to be much less time-consuming than the TFA approach. As for today, the critical drawback of TRA-MT is the forced choice between either sheathless 8F guide catheters, designed for femoral approach and carrying a higher risk of spasm/catheter entrapment [2], or 6F low-profile sheath and 6F guide catheters, with a significant downsizing of the aspiration catheter diameter [3]. Shortly after the new RED aspiration catheter family from Penumbra came to market in late 2022, it was noted that the thinnest one (RED 62) was fully compatible with a 7F guide catheter (e.g., Rist; Medtronic or Fubuki; Asahi Intecc Co., Ltd.). This combination was thought to be adequate for setting up a “radial ready” tricoaxial system that has very few differences compared to a conventional TFA approach (mainly a slight inner diameter gap with standard 6F aspiration catheters) and, at least in theory, is suitable even for large proximal large vessel occlusions (LVOs) [4]. In this study, we present our recent experience with this tricoaxial setup for patients who underwent MT for anterior circulation LVO via the TRA (TRA-MT).
MATERIALS AND METHODS
Study Design
This retrospective study included all consecutive patients with acute ischemic stroke (AIS) for anterior circulation LVO who received MT with a TRA (both first-line and bail-out after TFA) with the use of the same coaxial setup consisting in a 7F low profile hydrophilic sheath (Glidesheath Slender; Terumo), a radial-specific 7F guide catheter (Rist) and both 5.5F and 5F aspiration catheters (RED 62, Penumbra, Sofia 5F; MicroVention/Terumo and AXS Catalyst 5, Stryker Neurovascular), in a comprehensive stroke center between November 2022 and November 2023. The study was approved by the local ethics committee. Informed patient consent was waived due to the retrospective nature of this study. Diagnosis of AIS was made by computed tomography (CT), CT angiography (CTA), and CT perfusion; in case of no contraindications, intravenous lysis therapy (IVT) with recombinant tissue-type plasminogen activator was performed promptly after diagnosis after neurological assessment. The decision for MT was made in consensus by an interventional neuroradiologist and neurologist on service.
Endovascular Procedure
Data were retrospectively collected from a prospectively kept institutional MT database. Anterior circulation thrombectomies performed via the TRA, both as first-line and bailout in case of TFA approach failure, were analysed since the first use of the described setup. The choice of a first-line TRA approach was at the operator’s discretion, mainly in case of CTA recognition of a type III aortic arch in case of right-sided occlusion or the presence of left common carotid artery (CCA) origin from brachiocephalic trunk (the so-called, although a misnomer, “bovine arch”) for left-sided occlusions. No ultrasound measurement of radial artery diameter was performed before arterial puncture. After the insertion of the 7F-16 cm low-profile sheath (Glidesheath Slender), an intra-arterial bolus of 250 mcg of nitroglycerine was administered through the sheath’s sideport and an unsubtracted forearm high fps fluorography was obtained in order to check radial and brachial anatomy and assess a potential presence of spasm. The guide catheter (Rist) was then connected to a saline flush line with diluted nimodipine (2 mg/ L in saline) and navigated with a coaxial 5F Simmons diagnostic catheter and a 035 wire to reach the ascending aorta, and thereafter was used to selectively catheterize target carotid artery. After reaching a stable position (usually distal cervical internal carotid artery [ICA]) with a guide catheter, a diagnostic angiogram was performed to check for occlusion point and collaterals and to create a roadmap. The choice from all aspiration catheters compatible with Rist was made with respect to the vessel occlusion site; the RED 62 aspiration catheter (0.062” ID) was used mainly for ICA terminus or proximal M1 occlusions, while for distal M1-dominant M2, 5F aspiration catheters, such as Sofia 5F (0.055” ID) or Catalyst 5 (0.058”), were used. Thrombectomy was then performed in the usual fashion; the choice of microcatheter, microwire, and the additional use of stentriever was at the operator’s discretion. The interventional procedures were performed under general anaesthesia or conscious sedation as per internal protocol. A final angiographic run from radial sheath is performed immediately prior to hemostasis, performed with a Prelude hemostatic bracelet (Merit) inflated with 18 mL of air and immediately titrated to achieve patent hemostasis for usually 60 minutes. Fig. 1 shows a “standard” setup for TRA-MT.

Successful TRA-MT in a septuagenarian with a National Institutes of Health Stroke Scale score of 23, Alberta Stroke Program Early CT score of 8, and a CT angiography demonstration of right ICA apex occlusion associated with relevant hypoplasia of right A1 tract of anterior cerebral artery (A, B). Type II aortic arch (C). Radial artery fluoroscopy run showed no forearm tortuosities (D). After a straightforward right common carotid artery catheterization (E) digital subtraction angiography in anteroposterior view, unsubtracted, confirmed the ICA apex occlusion (F) A RED 62 aspiration catheter in combination with a large diameter stent-retriever (Aperio 6×50 mm; Acandis) was used to perform thrombectomy (G), resulting in thrombolysis in cerebral infarction score 3 after a single passage (H). TRA, transradial approach; MT, mechanical thrombectomy; CT, computed tomography; ICA, internal carotid artery.
Evaluation of Demographic, Technical, and Clinical Data
Demographic data included age, sex, onset-to-needle time, baseline modified Rankin scale (mRS) score, gravity of symptoms defined as National Institutes of Health Stroke Scale (NIHSS) score, preprocedural IVT, and baseline imaging characteristics (location and side of vessel occlusion).
Procedural data analysis included choice of TRA access as front-line or bail-out after failed TFA approach, time from wrist puncture to intracranial access (defined as the time between the first angiographic run from the radial sheath to the first intracranial run), the rate of crossover from radial to femoral access, the embolectomy technique (direct aspiration vs. combined technique with stentriever), and the number of thrombectomy passes. Technical outcome evaluation was based on the modified thrombolysis in cerebral infarction (mTICI) [5] score, with a rate of ≥mTICI 2b recanalization rate and final mTICI score.
NIHSS scores on admission and at discharge and the mRS score at 90 days post-procedure were assessed to identify any post-procedural neurologic deficits. The mRS score was obtained at 30 days after discharge. Favourable clinical outcomes were defined as mRS scores of 0-2. Follow-up CT scans were obtained 24 hours after treatment in all patients or earlier in cases of clinical deterioration. Intracranial hemorrhage was defined according to the Heidelberg classification. Radial artery occlusion (RAO) rate at 24 hours was assessed with the reverse Barbeau test, an indirect evaluation of radial artery patency [6].
Statistics
Categorical variables were reported as proportions. Continuous variables were reported as mean±standard deviation or median with interquartile range (IQR), as appropriate based on the data distribution. In each group, categorical variables were compared using the chi-squared test, and continuous variables were compared using the Kruskal–Wallis test. Two-sided P-values <0.05 were defined as statistically significant. All statistical analyses were performed using SPSS version 24.0.0.1 (IBM Co.).
RESULTS
Study Population and Baseline Characteristics
Between November 2022 and November 2023, 155 consecutive patients presented with AIS due to anterior circulation LVO and underwent endovascular thrombectomy. From a total of 24 patients who underwent TRA-MT, the aforementioned setup was used in 22 patients (14.2%), as a first-line approach in 19 patients (86.4%), and as a bail-out approach after failed TFA in 3 patients (13.6%). The rate of crossover from radial to femoral was 2/22 (9.1%), noting that those 2 patients underwent subsequent TFA-MT with a conventional 8F guide catheter and 6F aspiration catheter and were therefore excluded from the analysis. The causes of TFA crossover were a type 2 aortic arch with acute angle of brachiocephalic trunk and CCA origin from aortic arch (Fig. 2) and an antecubital loop of radial artery that was deemed to be extremely harmful to cross (Fig. 3). The new total consisted of 20 patients (9 males, 11 females) with a mean age of 77.8±12.5 years (range 33–91). The mean NIHSS score was 9.25±7.85 (range 4–28). Regarding occlusion site, 10/20 patients (50.0%) presented with occlusion of M1 segment of the middle cerebral artery (M1 MCA), 6/20 patients (30.0%) presented with occlusion of ICA terminus occlusion, and 4/20 patients (20.0%) presented with occlusion of M2 segment of the middle cerebral artery (M2 MCA); 12/20 (60.0%) were right-sided occlusions and 8/20 (40.0%) were left-sided. Detailed patient characteristics are shown in Table 1.
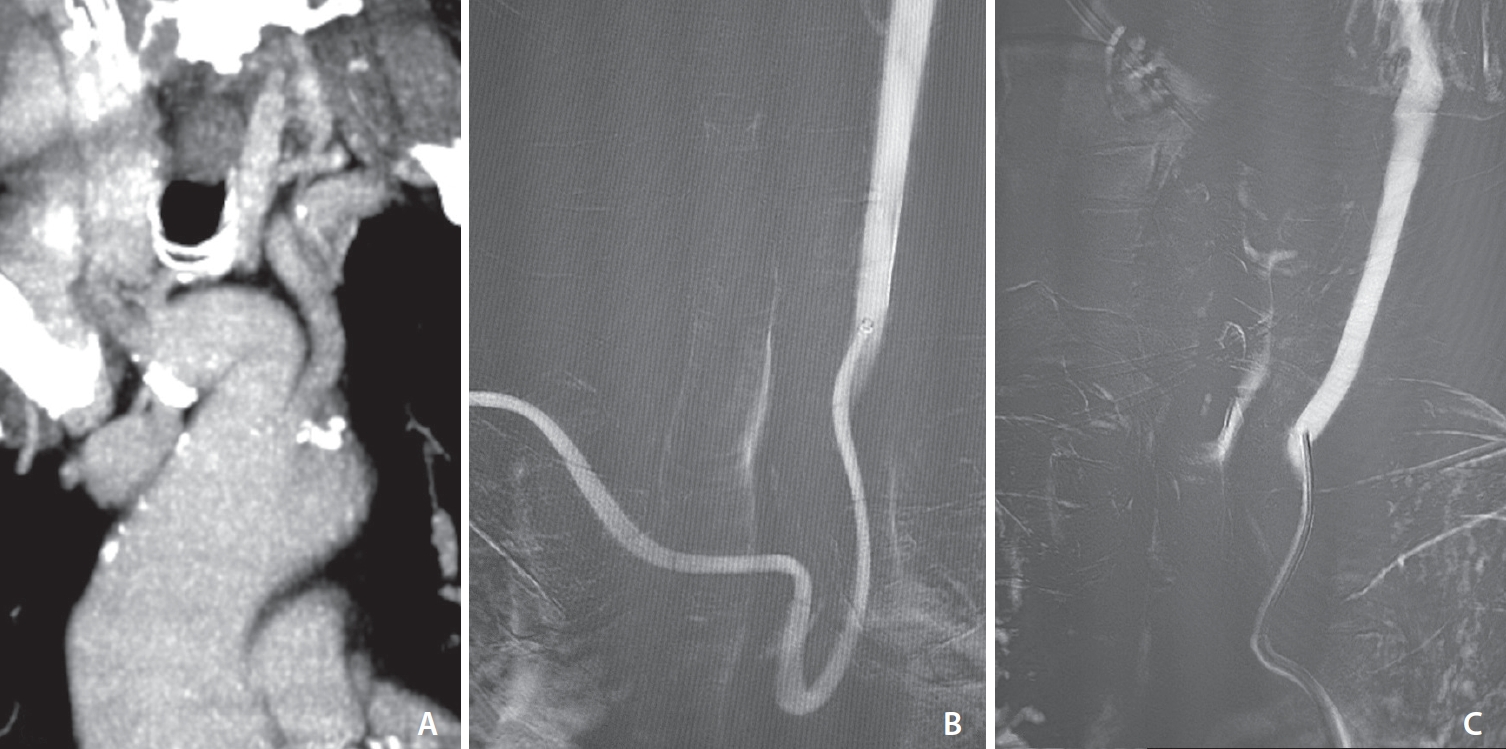
A case of unsuccessful TRA-MT, due to nonbovine left common carotid artery origin associated with acute angulation of proximal brachiocephalic trunk (A). Rist guide catheter couldn’t navigate further due to a tendency of prolapsing into the aortic arch (B). An 8F femoral access was obtained with straightforward catheterization of common carotid artery (C). TRA, transradial approach; MT, mechanical thrombectomy.
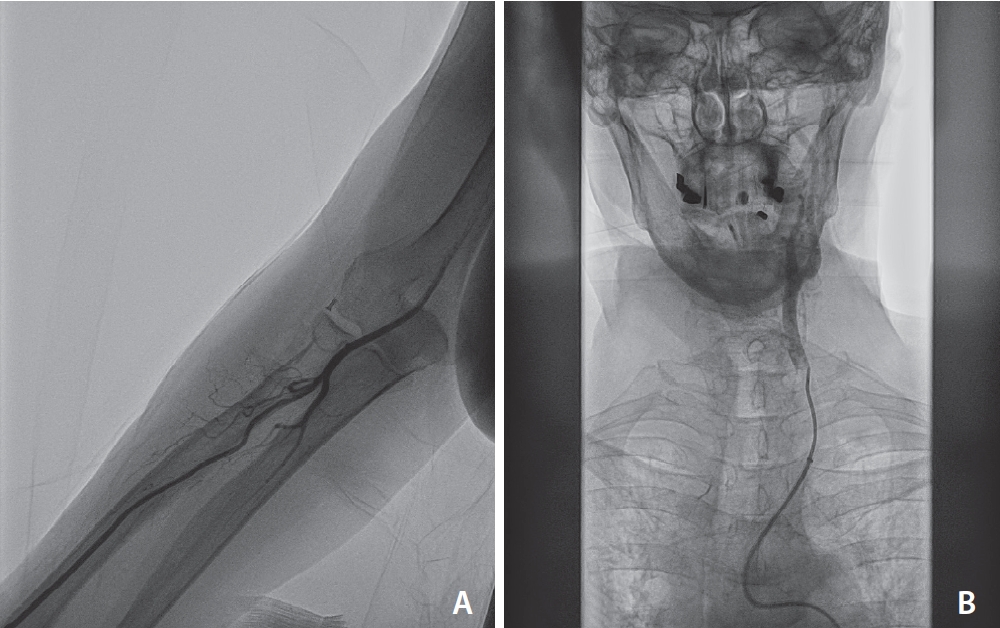
Another case of unsuccessful TRA-MT, due to a radial artery loop in antecubital region which was deemed to be harmful to cross (A). An 8F femoral access was obtained with straightforward catheterization of left common carotid artery (B). TRA, transradial approach; MT, mechanical thrombectomy.
Procedural Characteristics
The time from wrist puncture to intracranial access was 7.7±3.6 minutes (median 7 minutes, range 4–18). A direct aspiration, first pass technique (ADAPT) was performed in 6/20 cases (30.0%), a combination of aspiration and stentriever in 13/20 (65.0%), and thrombectomy with stentriever only in 1/20 (5.0%), for a M2 MCA occlusion. In case of proximal LVO (intracranial ICA, proximal M1; n=16), the RED 62 catheter was chosen in 100% of cases, while for distal LVO (distal M1, proximal dominant M2; n=4) Sofia 5 was chosen in 75.0% of cases, and in a single case thrombectomy was performed with stentriever only and distal access catheter (AXS Catalyst 5) in the proximal MCA. A mean of 1.6±0.9 passes was reported. In one case, an intracranial stent was placed after the first direct thromboaspiration pass because of the presence of a carotid apex dissection, also involving the proximal M1 segment of MCA. Final mTICI rates were ≥2b for 18/20 patients (90.0%) and ≥2c for 16/20 patients (80.0%). Further details are listed in Table 2.
Safety and Clinical Outcomes
Two procedural adverse events (10.0%) were observed in the study cohort, both hemorrhagic and thought to be procedure-related (stentriever maneuvers). In 1 of those cases, a massive bilateral subarachnoid haemorrhage (SAH) was observed after a single thrombectomy pass due to complete fibrinogen depletion in a patient who underwent IVT with an active bleeding wound. In the other case, a slight sylvian SAH was observed after 4 thrombectomy maneuvers, which remained asymptomatic and spontaneously resolved after 24 hours. Four patients (20%) showed a hemorrhage at 24 hours postprocedural CT scan, of which 2 (10.0%) were scored at hemorrhagic infarction type 1 (HI1) and 2 (10.0%) developed symptomatic parenchymatous hematoma type 2 (PH2), according to Heidelberg bleeding classification [7]. No other intracranial complications were reported. The overall in-hospital mortality rate was 2/20 (10.0%). As revealed by the Kruksal–Wallis test, NIHSS score showed a trend toward a reduction 24 hours after MT, although non-statistically significant (8.9±7.9 vs. 9.25±7.85; P=0.056), while at discharge NIHSS was significantly lower than at admission (8.65±9.06 vs. 9.25±7.85; P=0.027). Clinical follow-up was available for only 18 out of 20 patients; of them, mRS scores of 0-2 were achieved in 9 of the 18 patients (50.0%). No extracranial complications were reported; in particular, hemostasis was uneventful for all patients. Regarding radial artery patency, a reverse Barbeau test for RAO assessment was performed in 19 patients and the incidence of RAO was detected at 10.5% (2/19).
DISCUSSION
Direct TRA-MT for anterior circulation stroke is still considered an exception, adopted mainly as a bail-out technique in cases of failure of TFA catheterization of epiaortic vessels, with few data mainly derived from case reports or small case series [8-12]. The concerns are mainly related to the estimated slower time from puncture to ICA stable access than from the femoral artery and radial artery tortuosity at the forearm, which is relevant especially in older people [13]. Also, the forced choice between the use of an 8F guide catheter as long sheath (very high risk of radial artery spasm [RAS] and injury) or a combination of 6F sheath and guide catheter (significant downsizing of the aspiration catheter diameter: 5F maximum) could be perceived as a relevant drawback. These aspects could lead to, from the patient point-of-view, a worse outcome because of a lower reperfusion efficacy, and, from the operator point-of-view, a perception of disregard towards TRA access.
The use of the described setup has, in our experience, provided an optimal balance between trackability in the radial and brachial region, ease of epiaortic vessel catheterization, and intracranial reperfusion efficacy. The operative ease given by a tricoaxial system very similar to the one routinely used for TFA-MT allowed for a reasonable comfort even for a single-operator procedure and avoided the need of cumbersome exchanges for an 8F guide catheter as long sheath or the forced downsizing to a 6F guide catheter. The 7F low-profile sheath allows for the largest bore available while avoiding risk of RAS during initial maneuvers, and the 7F radial-specific guide catheter has a specific coating and transition zones dedicated for angulation in TRA catheterization of epiaortic vessels that offers increased compatibility with aspiration catheters, notably with the RED 62 (0.062” ID), allowing a high reperfusion rate even for large proximal LVOs (100% mTICI ≥2b in case of ICA apex occlusion). Munich et al. [12] also demonstrated the benefits of this setup, with a statistically significant reduction of puncture-to-recanalization time and an increase of mTICI ≥2b recanalization rate in comparison to a 6F sheath-6F guide catheter system. As shown in Table 1, intracranial catheterization time is always <20 minutes (median 7 minutes), which means a higher chance to get a quick and effective intracranial access, with no occurrence of critical RAS causing a procedure abortion. These favorable technical specifications could further lower the threshold to radial access in case either of a challenging craniocervical anatomy (especially bovine morphology for left-sided occlusion, type III aortic arch, or extensive aortoiliac atheromatous disease), or when the groin region is inaccessible due to pregnancy or severe obesity. Forearm issues such as radial artery tortuosity or loops can be managed effectively with specific techniques; in our experience, the only TRA abortion due to a radial loop occurred at the very early beginning of our experience with this setup. In case of left-sided occlusion, however, a type III aortic arch in combination with a non-bovine left CCA origin could pose relevant difficulties for left carotid catheterization due to the relative unease of gaining a stabile intracranial access, as the guide catheter tends very frequently to prolapse in ascending aorta; such a case is shown in Fig. 2. In those cases, TFA should be considered as a first-line approach; while for right-sided occlusions, there are virtually no anatomical limitations to TRA. In terms of safety concerns, the intracranial complication rate is substantially in line as reported in literature for TFA-MT, also because of the absence of technical differences in reperfusion techniques. Regarding arterial access site complications, apart from a 10% incidence of RAO (all clinically silent), we did not experience any hemorrhagic issues at the forearm, and hemostasis was promptly obtained in all cases despite the use of a 7F sheath, which has a similar outer diameter as for 6F guide catheters (2.67 mm for Neuron MAX vs. 2.79 mm for Glidesheath Slender). This is proven by a large amount of evidence in the invasive cardiology field, demonstrating a significantly lower site-access complication rate for radial access in comparison to femoral access [14].
This study is limited by its retrospective, single-center, and self-reported design and the relatively small number of patients included. Further, although the intracranial access setup was specifically chosen among others for the totality of thrombectomies, the study cohort has some heterogeneity regarding variety in recanalization strategies. Due to the lack of evidence-based recommendations, the choice of a radial access was left to the discretion of the performing neurointerventionalist, thus leading to a potential selection bias. Given also the absence of a clearly defined anatomy threshold for choosing frontline TRA-MT, a comparison with TFA-MTs performed with similar epiaortic anatomies was not possible; this latter aspect was thought to be beyond the scope of this study. However, this study cohort represents a “real-world” initial experience of this setup. Finally, any longterm clinical follow-up data were not included as the primary criteria were technical efficacy and safety profile. Future prospective and comparative studies in larger cohorts are needed especially to further characterize the optimal threshold for choosing the proper vascular access in order to achieve a fast, effective, and safe intracranial recanalization.
CONCLUSION
In conclusion, this study shows a high technical effectiveness of this specific tricoaxial setup for TRA-MT in AIS, even for large proximal LVOs, with a fast time from puncture to intracranial catheterization, a low TRA-to-TFA crossover, and a good safety profile. Further studies are needed in order to establish a more straightforward pathway in choosing between transradial vs. his TFA for MT procedures.
Notes
Fund
None.
Ethics Statement
Ethical approval obtained from the local institutional review board (243/2021); the board waived the need for patient consent. The paper does not include any images or information that may identify the person.
Conflicts of Interest
The authors have no conflicts to disclose.
Author Contributions
Concept and design: SM and MB. Analysis and interpretation: SM, FM, and GR. Data collection: SM, RR, FM, GR, and UAG. Writing the article: SM. Critical revision of the article: SM, RR, FM, UAG, and MB. Final approval of the article: SM, FM, and MB. Statistical analysis: SM, FM, and GR. Overall responsibility: SM.


